What Colors of Light Appear in a Continuous Spectrum
What is light?
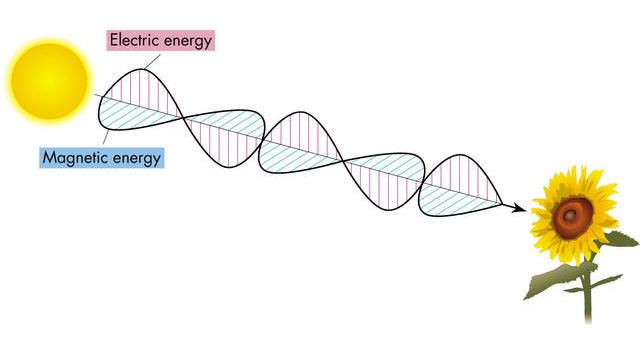
- Light is energy in the form of electromagnetic waves.
- Electric charge is the source of electric field lines and charges communicate through electric field lines.
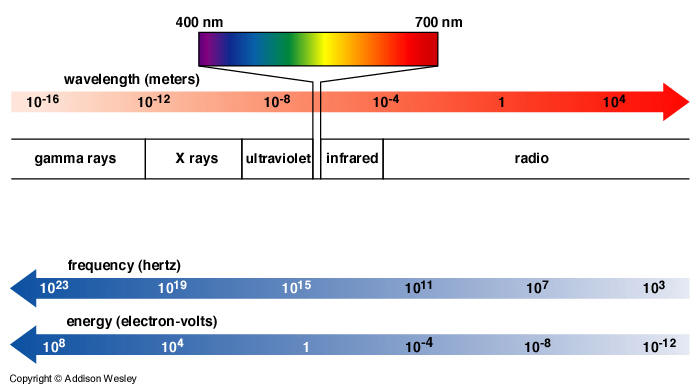
- The human eye can only detect a tiny sliver of the electromagnetic spectrum (400-700 nm).
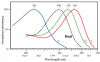
- White light can be considered a combination of all wavelengths. The colors seen when white light is passed through a prism and broken into a rainbow are:
Violet Indigo Blue Green Yellow Orange Red - Electromagnetic waves tend to interact with objects of roughly the same size as the wavelength.
- Short-wavelength radiation (UV, x-rays, gamma rays) is called ionizing radiation because it has the potential to ionize an atom or molecule and produce highly reactive species (radicals). These ionizations, if enough occur, can be destructive to biological tissue. If damage involves DNA, cancer can result.
- Long-wavelength radiation (visible light, microwaves, and radio waves) is called non-ionizing radiation because its wavelength is much bigger than atoms and molecules, which therefore cannot be chemically altered by this radiation. Long-wavelength radiation is not considered harmful to biological tissue.
- The atmosphere has "windows" which tend to filter out much of the harmful short-wavelength components.
- Visible light and radio waves mostly get through unimpeded.
- Infrared and ultraviolet are partly blocked.
- X-rays and gamma rays are completely blocked. Why is Lois Lane feeling sick?

![]()


Line vs Continuous
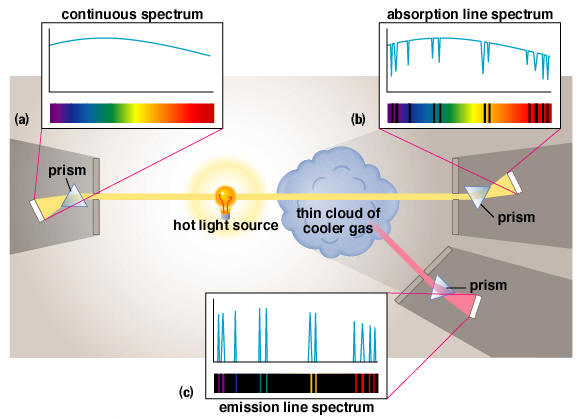
- Light which passes through a spectroscope produces 3 types of spectra:
- Continuous (rainbow) spectrum�produced by a hot, dense object. A continuous spectrum is also called blackbody radiation.
- Emission spectrum�produced by a hot, rarefied gas against a dark background.
- Absorption spectrum�produced by a cool gas against a background of a hot, dense object.
- For a line spectrum, the background determines whether the line spectrum is an emission or absorption spectrum.
- Density determines whether the spectrum is a line spectrum (low density) or a continuous spectrum (high density).
- Some examples:
- An incandescent light bulb produces a continuous spectrum because the source of the light is a metal filament (wire).
- A continuous spectrum emanates from the dark parts of the universe. The peak of this radiation corresponds to a temperature of about 2.7 degrees Kelvin. This radiation, known as Cosmic Background Radiation, represents the 'echo' of the original Big Bang and is considered an important piece of evidence supporting the Big Bang Theory.
- A fluorescent light produces an emission spectrum because the source of the light is an 'excited' gas.
- Stellar spectra are generally absorption spectra because some absorption is occurring in the relatively cool atmosphere of the Sun. The dark absorption lines in the spectrum of our Sun are called Fraunhofer lines.
![]()
Line spectra
- Metals (and other elements) produce a characteristic color when put into a flame.
- The light from any vaporized element produces its own unique set of lines when sent through a slit and then through a prism. This is the basis of spectroscopy.
- Each spectral line corresponds to an electronic transition from one energy level to another. Click here for an illustrative animation.
- Atomic hydrogen produces a series of characteristic line spectra in the ultraviolet, visible, and infrared parts of the total spectrum.
- The visible light spectra of hydrogen always consist of two violet lines, a blue-green line, and a bright red one.
- Line strength is not only a function of source concentration (more atoms, more signal) but is also a strong function of temperature. A star with a surface temperature of 6000 K, for instance, will exhibit a stronger signal for calcium than for hydrogen, even though it has a lot more hydrogen.
- Because of their strong spectroscopic dependence on temperature, stars are classified on the basis of temperature.
- Spectral lines can also be broadened by several factors:
- rotation--one side of a star is red-shifted, the other is blue shifted (Doppler broadening)
- magnetic fields--which "smear" energy levels
- increasing density or pressure in the gas--this again "smears" energy levels









Doppler effect
- The Doppler effect applies to all waves, including sound and light.
- This video might be helpful in visualizing the Doppler effect.
- The Doppler effect is the apparent shift in wavelength (or frequency) of light (or any other wave) due to the relative motion of source and observer:
- Red Shift : The distance between the observer and the source is increasing.
- Blue Shift : The distance between the observer and the source is decreasing.
- Distant galaxies are moving away from us (and from each other) and are thus red-shifted (see Big Bang page).

What can we learn by analyzing starlight?
- A star�s temperature
- by peak wavelength (Wien's law). Wien's laws states that temperature and peak wavelength are inversely related. As one gets bigger by some factor, the other gets smaller by the same factor.
- strength of spectral lines
- A star�s chemical composition
- by spectral line analysis
- A star's motion
- A star�s radial velocity from Doppler shifts
- A star's orbital velocity from the Doppler
- A star's rotational rate from Doppler line broadening
- A star's orbital period from dips in the luminosity curve of binaries; click here for an animation.




Vision and color perception
- Human eyes have a light-sensitive layer--the retina--which carries two types of light-sensitive cells: rods and cones.
- Rods are sensitive to dim light (scotopic vision) but do not provide color information. Rods are responsible for vision at night (scotopic vision), but offer very poor resolution (about the same as 20/200 vision in daylight).
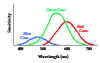
- Cones respond only to relatively bright light but relay information about 3 colors--red, green and blue. These are the so-called primary colors.
- The light response of the rods peaks sharply in the blue-green (507 nm). Rods respond very poorly to red light.
- Bright sunlight is about one billion times brighter than the dimmest light in which the rods can operate.
- For greatest acuity look straight; for greatest sensitivity, look to the side.
- Rods and cones are not evenly distributed on the retina. Cones are concentrated centrally, in an area called fovea, while rods more concentrated away from the center.
- Any visual task that requires seeing fine detail (e.g., reading the letters on this webpage) uses the fovea, a small area on the retina with only cones and no blood. The fovea sees only the central two degrees of the visual field, roughly equivalent to twice the width of your thumbnail at arm's length. At night, the fovea becomes the "night blind spot".
- To see a very faint object, we need to involve as many rods as possible. Rods are largely responsible for peripheral vision and are more sensitive to dim light and motion. The most sensitive portion of the eye usually turns out to be 8-16 degrees away from the center. (The fovea contains only cone cells, which serve as bright light and color detectors but are not as useful in night vision.) Looking a bit with the side of the eye, a technique known as averted vision, is very useful to astronomers, as it often allows them to see especially faint or otherwise invisible objects. The most effective direction is that which places the object on the nasal side of the vision. Some observers report a gain of up to 3-4 magnitudes. There is some evidence that the technique has been known since ancient times.
- In a similar technique, known as scope rocking, the telescope is moved back and forth slightly to move the object around in the field of view. This technique is based not only on the greater density of rods away from the center of the eye but also on the fact that rods are more sensitive to motion than to static objects.
- Honeybees and bumblebees have trichromatic (i.e., with 3 color-sensitive receptors) color vision, which is insensitive to red but sensitive in ultraviolet to a color called bee purple.
- Snakes are more sensitive to infrared.



Color Arithmetic
- Some objects appear a certain color because they emit light with that color (or combination wavelengths). Other objects appears a certain color because they reflect that color.
- A red light is red because it emits red light.
- A red apple appears red in white light (which is a combination of red, green, and blue) because it reflects red light and absorbs (much of) the other colors (green and blue).
- In blue light, the apple would appear black because it absorbs blue and no light would therefore bounce off the apple.
- Similarly, the green leaves reflect green and absorb the other colors. The leaves would therefore appear green in green or white light and would appear black in blue or red light.
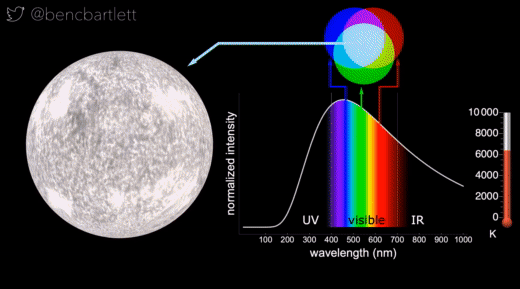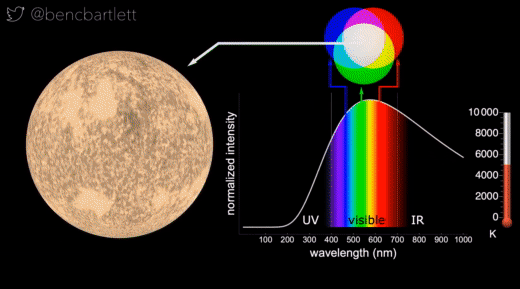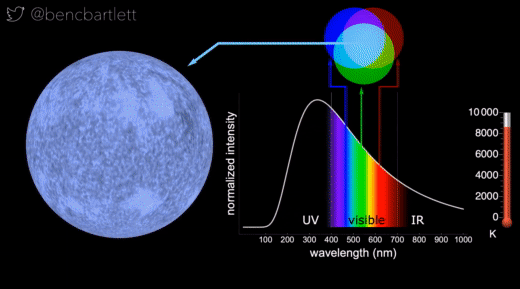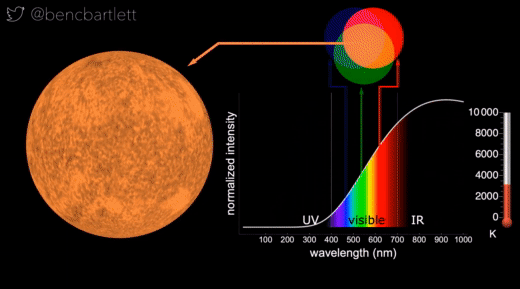
- The apparent color of stars depends on the relative intensity of the various wavelengths.
- Bright cool stars appear red because they have a lot more intensity on the red end of the spectrum than on the blue end.
- Bright hot stars appear blue because they have a lot more intensity on the blue end of the spectrum than on the red end.
- Dim stars, irrespective of wavelength distribution appear white because only the rods are active in dim light.
- Stars are never green because if there is enough intensity in the green (middle) part of the spectrum, there is generally enough in the neighboring bluish and reddish, so the perception is white.
- This video should be a nice visual of these considerations.
- Click here for an applet on color arithmetic to get a feel for the different color possibilities.

Return to class notes TOC.
Page last modified:
Source: https://people.highline.edu/iglozman/classes/astronotes/light.htm
0 Response to "What Colors of Light Appear in a Continuous Spectrum"
Post a Comment